
Understanding the Role of Amylin in Obesity
as an Emerging Therapeutic Target
This educational resource is provided by and supported by an educational grant from Novo Nordisk, Inc.
Explore cutting-edge knowledge of amylin biology and its therapeutic implications. Review this resource to learn about amylin as well as 3 other hormones—glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), and glucagon—and their various actions throughout the body.
Amylin

Amylin is a 37-amino-acid, neuroendocrine peptide hormone co-secreted with insulin by pancreatic beta-cells in response to nutrient intake. Though it has a short half-life, amylin is a multifunctional hormone that may play a significant role in metabolic regulation.
There are at least 3 amylin receptors—AMY1, AMY2, and AMY3—each of which is composed of the calcitonin receptor bound to 1 of 3 corresponding receptor activity-modifying proteins. Amylin receptors are considered proven drug targets, and amylin mimetics are emerging as novel treatments for overweight, obesity, and diabetes mellitus.
https://www.youtube.com/watch?v=jviZOW0qNzQ&feature=youtu.be
Click to watch the video Amylin Adventure: Biology and Modes of Action
Amylin is a multifunctional hormone through its actions on various organs:

Your Content Goes Here




Glucagon-like peptide-1 (GLP-1)
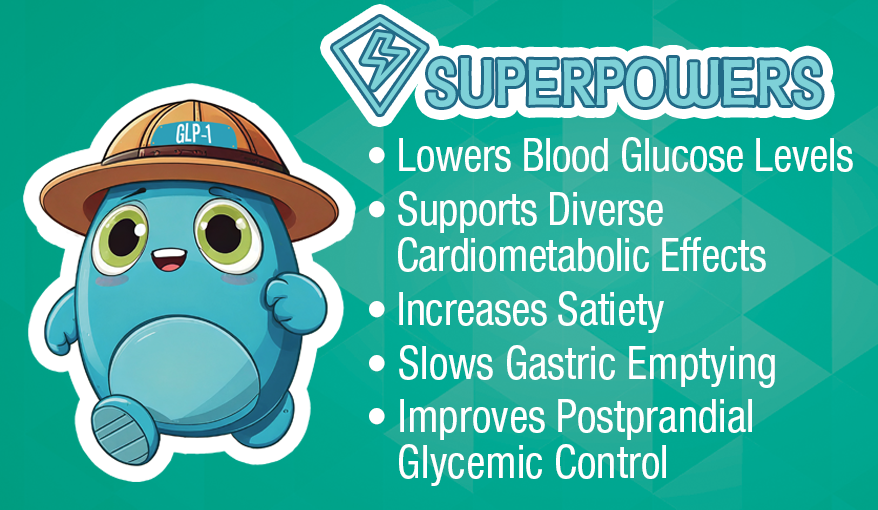
GLP-1 is a 30-amino-acid, incretin hormone secreted by intestinal L-cells in response to nutrient intake. It enhances glucose-dependent insulin secretion, inhibits glucagon release, delays gastric emptying, and promotes satiety.
Many professional organization and medical societies recognize approved GLP‑1 receptor agonist-based obesity medications, including as semaglutide and tirzepatide, as highly-effective for treating excess adiposity in adults with obesity or overweight and related complications.
Guidance from the American Diabetes Association (ADA), American Association of Clinical Endocrinology/The Obesity Society (AACE/TOS), American Gastroenterological Association (AGA), Endocrine Society, and the American Heart Association (AHA) notes that large‑scale trials of these agents have shown sustained weight loss (roughly 10% to 20%), as well as improvements in glycemic control, blood pressure, lipid profiles, and other cardiometabolic risk factors.
Reflecting this evidence, clinical guidelines increasingly support GLP-1 receptor agonists as an appropriate first-line or add-on treatment option when obesity pharmacotherapy is indicated, in conjunction with comprehensive lifestyle interventions.
GLP-1 exerts a broad range of physiological effects due to the widespread expression of GLP-1 receptors in various organs:


Your Content Goes Here




Glucose-dependent insulinotropic polypeptide (GIP)

GIP is a 42-amino-acid incretin hormone secreted by enteroendocrine K-cells in the upper small intestine in response to nutrient intake. GIP potentiates glucose-dependent insulin secretion and plays a role in postprandial glucose and lipid metabolism.
GIP affects weight and obesity through its insulinotropic effects on several organs:

Your Content Goes Here




Glucagon

Glucagon is a 29-amino-acid peptide hormone that is secreted by pancreatic alpha-cells.
It primarily functions to increase blood glucose levels by stimulating glycogenolysis and gluconeogenesis in the liver. It reduces hepatic lipogenesis and promotes fat oxidation, which can help reduce hepatic steatosis.
Glucagon is a counter-regulatory hormone to insulin, thus playing a crucial role in glucose homeostasis and metabolism. Glucagon’s primary actions are through the liver and pancreas.




Your Content Goes Here

These 4 hormones—amylin, GLP-1, GIP, and glucagon—interact in complex ways to regulate body weight and impact obesity through their actions on the brain, stomach, adipose tissue, pancreas, bone, and liver.

In the context of obesity, amylin’s ability to enhance satiety and reduce food intake works synergistically with the other hormones.
- Like GLP-1, amylin also promotes satiety and reduces food intake.
- Amylin inhibits glucagon postprandially to prevent excessive hepatic glucose production.
- GIP primarily promotes insulin secretion and fat storage. However, amylin’s satiety effects can counteract GIP’s adipogenic actions, thus aiding in weight management.
In summary, these hormones interact to regulate weight and obesity by modulating insulin and glucagon secretion, influencing gastric emptying, and acting on the central nervous system to control appetite and energy expenditure.
Their combined effects on various organs contribute to their overall impact on weight regulation and metabolic homeostasis.
About
Contact Us
For General inquiries, contact:
[email protected]
For Technical questions, contact:
[email protected]
For CME questions, contact:
[email protected]
Vindico Medical Education
6900 Grove Road
Building 100
Thorofare, NJ 08086-9447 USA
888-960-0256; 856-994-9400
FAX: 856-384-6680

Vindico Medical Education, LLC is accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC) to provide continuing education for the healthcare team through November 30, 2026.

Legal


